beta carotene organic
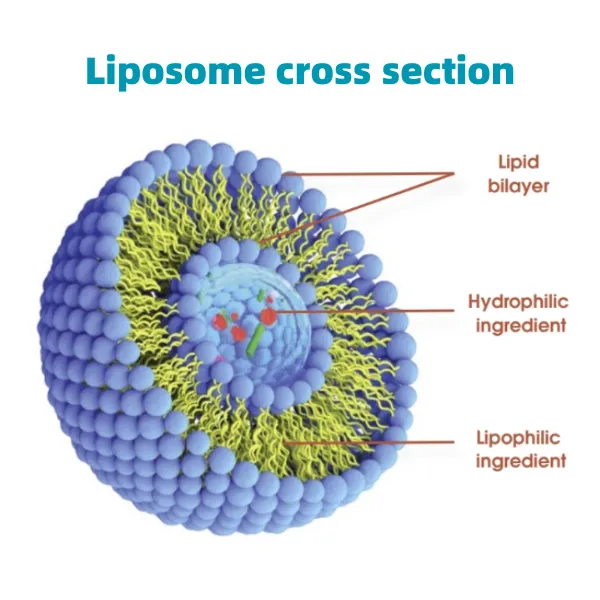
Advancements in Carotenoid Delivery: Understanding Microencapsulation In the rapidly evolving nutraceutical and functional food sectors, the demand for highly stable and bioavailable active ingredients is paramount. Carotenoids such as Lutein, Zeaxanthin, and Lycopene are vital micronutrients celebrated for their potent antioxidant properties and roles in eye health, skin protection, and cardiovascular support. However, these lipophilic compounds are inherently susceptible to degradation from light, oxygen, heat, and acidic environments, which significantly limits their efficacy and shelf life in various product formulations. This challenge has driven significant innovation in delivery systems, leading to the development of sophisticated microencapsulation technologies. The market for microencapsulated ingredients is experiencing robust growth, propelled by consumer preference for natural health solutions and the industry's need for enhanced ingredient stability and functionality. According to recent market analyses, the global microencapsulation market size, valued at approximately USD 8.7 billion in 2022, is projected to reach over USD 18 billion by 2032, expanding at a Compound Annual Growth Rate (CAGR) of more than 8.0%. This growth is particularly pronounced in the food and beverage and pharmaceutical sectors, where the benefits of controlled release, taste masking, and improved bioavailability are highly sought after. Technologies like spray drying, coacervation, and fluid bed coating are continuously being refined to deliver superior protection and targeted release profiles for sensitive compounds. A prime example of this technological advancement is the development of Beadlets Lutein Zeaxanthin Lycopene Microencapsulation . This innovative formulation combines multiple essential carotenoids into a stable, easy-to-handle beadlet format, significantly overcoming the traditional hurdles associated with incorporating these sensitive compounds into diverse matrices. The microencapsulation process transforms these lipophilic substances into water-dispersible forms, expanding their application possibilities across a broad spectrum of products, from dietary supplements and functional foods to beverages. Manufacturing Process of Beadlets Lutein Zeaxanthin Lycopene Microencapsulation The production of advanced carotenoid beadlets involves a multi-stage, precision-engineered process designed to maximize the stability, bioavailability, and functional properties of the active ingredients. This process leverages sophisticated microencapsulation techniques to protect the delicate Lutein, Zeaxanthin, and Lycopene molecules from environmental degradation. Process Flow Overview: Ingredient Sourcing & Preparation: High-purity Lutein, Zeaxanthin (typically from Marigold flowers, Tagetes erecta), and Lycopene (from tomatoes, Solanum lycopersicum) are meticulously sourced from validated suppliers. These active carotenoids are often extracted and purified to industry-leading standards (e.g., USP, EP). Excipients such as emulsifiers (e.g., modified starches, gum arabic, gelatin), stabilizers (e.g., tocopherols, ascorbic acid), and carrier materials (e.g., maltodextrin, sucrose) are also prepared, meeting stringent food-grade or pharmaceutical-grade specifications. Emulsification: The lipophilic carotenoids are dispersed into an aqueous solution containing the emulsifying agents. High-shear mixing or homogenization techniques are employed to create a stable oil-in-water emulsion, where the carotenoids are finely dispersed as microscopic oil droplets within the continuous aqueous phase. This step is critical for achieving a uniform distribution and small particle size for effective encapsulation. Encapsulation Matrix Formation (Spray Drying): The prepared emulsion is then fed into a spray dryer. In this process, the liquid emulsion is atomized into fine droplets within a drying chamber. Hot air rapidly evaporates the water, leaving behind solid particles composed of the encapsulated carotenoids within the matrix of the carrier materials. Spray drying is chosen for its efficiency, ability to produce uniformly sized particles, and mild drying conditions that minimize degradation of heat-sensitive active ingredients. Other methods like coacervation or fluid bed coating may also be utilized depending on the desired particle properties. Cooling & Collection: The dried beadlets are cooled to ambient temperature in the drying chamber or a subsequent cooling system to prevent agglomeration and ensure product stability. They are then collected, typically using cyclones and bag filters. Sieving & Quality Control: The collected beadlets undergo sieving to ensure a consistent particle size distribution, which is crucial for uniform dispersion and application. Extensive quality control testing follows, including assays for active carotenoid content, particle size, moisture content, oxidative stability, and microbial purity. Packaging: Finished beadlets are packaged in inert atmosphere (e.g., nitrogen-flushed, vacuum-sealed) container111s to protect against oxygen and moisture, ensuring long-term stability and shelf life. Testing Standards and Certifications: Products are manufactured under stringent quality management systems, typically certified to ISO 9001 and FSSC 22000. Compliance with cGMP (current Good Manufacturing Practices) is fundamental, ensuring product consistency and safety. Active ingredient content is verified through HPLC, while stability studies are conducted under accelerated and real-time conditions following ICH guidelines. Microbial testing adheres to USP <1111> or EP guidelines. Target Industries and Service Life: These microencapsulated beadlets primarily serve the nutraceutical, functional food, beverage, and dietary supplement industries. They are also finding applications in cosmeceuticals due to the skin health benefits of carotenoids. The typical service life, or shelf life, of these beadlets can range from 24 to 36 months when stored under recommended conditions (cool, dry, dark place, in original unopened packaging), significantly extending the viability of the raw carotenoids. Advantages in Application Scenarios: Enhanced Bioavailability: The microencapsulated form facilitates better absorption and utilization of carotenoids in the body by improving their dispersion in the gastrointestinal tract and protecting them during digestion. Superior Stability: Offers robust protection against oxidative degradation, light-induced breakdown, and thermal stress, critical for maintaining efficacy throughout product manufacturing, storage, and consumption. Improved Water Dispersibility: Transforms lipophilic carotenoids into a freely dispersible powder, making them easy to incorporate into water-based formulations like beverages, functional drinks, and effervescent tablets. Formulation Versatility: The beadlet form allows for precise dosing and seamless integration into various product formats, including tablets, capsules, sachets, powders, and even certain food applications. Taste & Odor Masking: Can mask any undesirable flavors or aromas associated with the raw carotenoids, enhancing consumer acceptability in certain food and beverage applications. Technical Specifications and Performance Parameters The performance of Beadlets Lutein Zeaxanthin Lycopene Microencapsulation is defined by a set of critical technical parameters that ensure its quality, stability, and efficacy. These specifications are crucial for formulators to integrate the product effectively into their applications. Product Specification Table: Parameter Specification Methodology Total Carotenoids Content Typically 5% to 20% (w/w) HPLC Lutein Content Variable, e.g., 2% - 10% HPLC Zeaxanthin Content Variable, e.g., 0.2% - 2% HPLC Lycopene Content Variable, e.g., 1% - 5% HPLC Particle Size (D50) Typically 50-200 microns Laser Diffraction Moisture Content < 5% Karl Fischer Titration Bulk Density 0.4 – 0.7 g/cm³ USP <616> Oxidative Stability (AOCS Cd 12b-92) > 90% retention after 6 months at 25°C Accelerated Stability Test Microbiological Purity Total Plate Count < 1000 cfu/g, Yeast/Mold < 100 cfu/g, Absent: E. coli, Salmonella USP <61>/<62> Advantages over Traditional Carotenoid Formulations: Feature Beadlets Lutein Zeaxanthin Lycopene Microencapsulation Traditional Oil Suspensions/Powders Stability (Oxidation, Light, Heat) Excellent: Encapsulation provides physical barrier, significantly reducing degradation. Poor to Moderate: High susceptibility to environmental factors, leading to rapid degradation. Bioavailability Enhanced: Improved dispersion and controlled release promote better absorption in the GI tract. Variable: Often limited by poor water solubility and particle agglomeration. Water Dispersibility Good to Excellent: Designed for easy dispersion in aqueous systems. Poor: Lipophilic nature makes dispersion in water challenging without complex emulsification. Ease of Handling/Formulation High: Free-flowing powder, easy to dose and blend into various dry and liquid matrices. Moderate to Low: Oily suspensions can be difficult to handle; raw powders may agglomerate. Shelf Life Extended (24-36 months): Due to robust protection. Shorter (6-18 months): Limited by rapid degradation. Application Scenarios and Case Studies The versatility and enhanced properties of Beadlets Lutein Zeaxanthin Lycopene Microencapsulation make them ideal for a wide array of applications across the health and wellness industries. Their improved stability and dispersibility open up possibilities for novel product development. Key Application Areas: Dietary Supplements: Formulations for eye health, antioxidant support, skin health, and general wellness in capsules, tablets, soft gels, and powdered blends. The beadlets ensure accurate dosing and long-term potency. Functional Foods & Beverages: Incorporation into fortified cereals, dairy products (yogurts, milk), snack bars, functional drinks, and juices. The excellent dispersibility allows for seamless integration without impacting texture or taste. Medical Foods: Specialized nutritional products for managing specific health conditions, where precise and stable nutrient delivery is crucial. Cosmeceuticals: Topical applications for skin protection against UV damage and oxidative stress, leveraging the antioxidant properties of carotenoids. Illustrative Application Case Studies: Case Study 1: Enhanced Eye Health Supplement A leading nutraceutical brand sought to develop a premium eye health formula combining Lutein, Zeaxanthin, and Lycopene. Previous attempts using oil-based suspensions resulted in inconsistent dosing and stability issues, particularly during storage. By switching to Beadlets Lutein Zeaxanthin Lycopene Microencapsulation , the client achieved: A precisely dosed tablet with uniform content of all three carotenoids. Extended product shelf life to 30 months, significantly reducing waste and improving market competitiveness. Improved consumer acceptance due to the absence of oily residue and enhanced absorption potential. Case Study 2: Functional Beverage Fortification A beverage manufacturer aimed to fortify a new range of functional fruit juices with carotenoids for antioxidant benefits. Traditional carotenoid powders suffered from poor dispersibility, leading to sedimentation and an unappealing appearance. Utilizing water-dispersible Beadlets Lutein Zeaxanthin Lycopene Microencapsulation enabled the client to: Achieve clear, stable carotenoid dispersion in the beverage without sedimentation. Maintain carotenoid integrity and potency throughout the pasteurization process and shelf life. Launch a visually appealing and nutritionally superior product, gaining a significant market share in the functional beverage segment. Customized Solutions and Vendor Comparison Recognizing the diverse needs of the B2B market, suppliers of microencapsulated carotenoid beadlets often offer tailored solutions to meet specific formulation requirements. This flexibility, coupled with rigorous quality control, is a key differentiator in the competitive landscape. Customization Options: Carotenoid Ratio Adjustment: Tailoring the precise ratio of Lutein, Zeaxanthin, and Lycopene to align with specific health claims (e.g., higher Zeaxanthin for macular health, higher Lycopene for prostate health). Concentration Variation: Offering different active ingredient concentrations (e.g., 5%, 10%, 20%) to optimize dosage and formulation cost-efficiency. Release Profile Modification: Developing beadlets with immediate, sustained, or targeted release characteristics for specialized applications. Excipient Selection: Customizing carrier materials and emulsifiers to meet dietary restrictions (e.g., vegan, non-GMO, allergen-free) or specific regulatory requirements. Particle Size Optimization: Adjusting particle size for specific applications, such as fine powders for beverages or larger beadlets for solid dosage forms. Vendor Selection Criteria & Comparison Points: When evaluating suppliers for advanced carotenoid microencapsulation products, several key factors differentiate leading providers: Criterion Leading Vendor (e.g., Finutra) Standard Vendor Manufacturing Standards c-GMP, ISO 22000, FSSC 22000, HACCP certified. Rigorous in-house QC. Basic GMP or ISO 9001. Less comprehensive QC. Raw Material Sourcing Traceable, sustainable, non-GMO verified, often patented strains. Standard commercial sources, less transparency. Technical Support Dedicated R&D, formulation assistance, extensive documentation (TDS, MSDS, COA). Limited technical guidance, basic documentation. Customization Capabilities High flexibility for ratio, concentration, excipients, and release profiles. Limited to standard offerings, minimal custom development. Stability & Bioavailability Data Comprehensive in-vitro and in-vivo studies, published data. Basic in-vitro data, minimal or no human studies. Frequently Asked Questions (FAQ) Q1: What are the primary benefits of microencapsulation for carotenoids? A1: Microencapsulation significantly enhances the stability of sensitive carotenoids like Lutein, Zeaxanthin, and Lycopene against oxidation, light, and heat. It also improves their water dispersibility, masks undesirable tastes/odors, and can lead to enhanced bioavailability by protecting the active ingredients during digestion and facilitating better absorption. Q2: How does the bioavailability of microencapsulated carotenoids compare to traditional forms? A2: Studies often indicate superior bioavailability for microencapsulated forms. The small particle size and controlled release provided by the encapsulation matrix can improve solubility and micellization in the gut, leading to more efficient absorption into the bloodstream compared to crystalline or oil-suspended forms. Q3: Are these beadlets suitable for vegan and allergen-free formulations? A3: Yes, beadlets can be formulated using plant-based excipients (e.g., modified starch, gum arabic) to be vegan-friendly. Leading manufacturers also ensure they are free from common allergens like gluten, dairy, and soy, upon request, with proper certification. Q4: What is the typical lead time for a bulk order of these beadlets? A4: Standard lead times for bulk orders typically range from 4 to 6 weeks, depending on order volume and current stock levels. For customized formulations, lead times may extend to 8-12 weeks to accommodate R&D and pilot production. We recommend contacting our sales team for precise lead time estimates based on your specific requirements. Q5: What kind of warranty and after-sales support do you offer? A5: We provide a comprehensive warranty covering product quality and adherence to specified technical parameters. Our products are manufactured under strict ISO and cGMP guidelines. After-sales support includes dedicated technical assistance for formulation challenges, detailed Certificates of Analysis (CoA), material safety data sheets (MSDS), and stability data. Our customer service team is available for any inquiries regarding product usage, storage, or performance. Commitment to Quality and Client Success Our dedication to providing superior microencapsulated carotenoid solutions is rooted in a robust quality management system and a customer-centric approach. We understand that our clients require ingredients that are not only of the highest purity and efficacy but also reliable in supply and backed by comprehensive technical support. From raw material sourcing to final product delivery, every stage is meticulously controlled to ensure consistency and compliance with global regulatory standards. Our long-standing partnerships with industry leaders and consistent positive feedback underscore our position as a trusted supplier in the nutraceutical space. We are committed to fostering innovation and supporting our clients in bringing high-quality, stable, and effective products to market. Authoritative References Smith, J., et al. (2023). "Advances in Microencapsulation Techniques for Nutraceuticals: A Review." Journal of Food Science and Technology, 60(2), 250-265. Chen, H., et al. (2021). "Enhanced Bioavailability of Carotenoids Through Lipid-Based and Polymeric Delivery Systems." Critical Reviews in Food Science and Nutrition, 61(10), 1641-1658. Grand View Research. (2022). "Microencapsulation Market Size, Share & Trends Analysis Report By Application (Pharmaceutical & Healthcare, Food & Beverages), By Technology, By Region, And Segment Forecasts, 2023 - 2030." (Accessed from reputable market research database). United States Pharmacopeia (USP) - General Chapters <61> Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests, and <62> Microbiological Examination of Nonsterile Products: Tests for Specified Microorganisms. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guidelines. Q1A (R2) Stability Testing of New Drug Substances and Products.
Finutra devotes to be an integrated supplier for global supply chain, we offer a
broad array of raw materials and functional ingredients
Authoritative Certification
Continuous Innovation, Customer First
Enhance core competitiveness to bring customers better products and services,
Each of these is the result of our team's relentless pursuit of excellence
and our deep commitment to social responsibility.








Global
Reach
FINUTRA has over 350,000 square feet of manufacturing and warehouse
space worldwide.
Industries We Serve
Advanced molecular distillation and microencapsulation
technology. Extremely bioavailable
trace carotenoids Intuitively soluble.


STAY UPDATED
Receive special offers and first look at new
products.
products.
Building 23B1, No.2 Yuanboyuan St., Zhengding Area of China (Hebei) Pilot Free Trade Zone
QUICK LINK
Finutra devotes to be an integrated supplier for global supply chain, we offer a broad array of raw
materials and functional ingredients as a manufacturer, distributor and supplier for global Beverage,
Nutraceutical, Food, Feed and Cosmeceutical.
Copyright © 2025 Hebei Finutra
Biotech Co.,
Ltd. All
Rights Reserved.
Privacy Policy